For the use of a Registered Medical Practitioner or a Hospital or a Laboratory only
Sodium Alginate and Potassium Bicarbonate Chewable Tablets (500mg+100mg)

GENERIC NAME
Sodium Alginate & Potassium Bicarbonate Chewable Tablets (500mg + 100mg)
QUALITATIVE AND QUANTITATIVE COMPOSITION
Each uncoated chewable tablet contains:
Sodium Alginate IP ..................... 500 mg
Potassium Bicarbonate BP ......... 100 mg
Colour: Quinoline Yellow
DOSAGE FORM AND STRENGTH
Uncoated Chewable Tablet
CLINICAL PARTICULARS
THERAPEUTIC INDICATION
Treatment of symptoms resulting from the reflux of acid, bile and pepsin into the oesophagus such as acid regurgitation, heartburn, indigestion (occurring due to the reflux of stomach contents), for instance, after gastric surgery, as a result of hiatus hernia, during pregnancy, accompanying reflux oesophagitis, including symptoms of laryngopharyngeal reflux such as hoarseness and other voice disorders, sore throats and cough. It can also be used to treat the symptoms of gastro-oesophageal reflux during concomitant treatment with or following withdrawal of acid suppressing therapy
Posology and method of administration
Adults and children 12 years and over: One to two tablets after meals and at bedtime.
Children under 12 years: Should be given only on medical advice.
Elderly: No dose modifications necessary for this age group.
Hepatic Impairment: No dose modification necessary.
Renal Insufficiency: Caution if highly restricted salt diet is necessary
Method of administration: For oral administration only
The tablet must be chewed thoroughly before swallowing
CONTRAINDICATIONS
This medicinal product is contraindicated in patients with known or suspected hypersensitivity to the active substances or to any of the excipients.
SPECIAL WARNINGS AND PRECAUTIONS FOR USE
If symptoms do not improve after 7 days, the clinical situation should be reviewed
This medicinal product contains 53.22 mg sodium per tablet, equivalent to 2.7% of the WHO recommended maximum daily intake for sodium.
Each two-tablet dose contains 200 mg (2.0 mmol) of calcium carbonate. Care needs to be taken in treating patients with hypercalcaemia, nephrocalcinosis and recurrent calcium containing renal calculi.
DRUG INTERACTION
A time-interval of 2 hours should be considered between this formulation intake and the administration of other medicinal products, especially tetracyclines, fluoroquinolones, iron salts, thyroid hormones, chloroquine, bisphosphonates, and estramustine. Alcohol consumption makes the stomach produce more acid, which can further lead to heartburn. Therefore, avoid alcohol consumption.
USE IN SPECIAL POPULATION
Fertility, pregnancy and lactation Pregnancy:
Clinical studies in more than 500 pregnant women as well as a large amount of data from post-marketing experience indicate no malformative nor foeto/neonatal toxicity of the active substances. This drug can be used during pregnancy, if clinically needed
Breast feeding:
No known effect on breast fed infants. This drug can be used during breast feeding.
Fertility:
No known effect on human fertility
EFFECTS ON ABILITY TO DRIVE AND USE MACHINES
None
UNDESIRABLE EFFECTS
Adverse reactions have been ranked under headings of frequency using the following convention: very common (1/10), common (1/100 and < 1/10,000) uncommon (1/1000 and 1/100), rare (1/10,000 and < 1/1000), very rare (<1/10,000) and not known (cannot be estimated from the available data)
| System Organ Class | Frequency | Adverse Event |
|---|---|---|
| Immune System Disorders | Very rare | Anaphylactic and anaphylactoid reactions. Hypersensitivity reactions such as urticaria |
| Respiratory, Thoracic and Mediastinal Disorders | Very rare | Respiratory effects such as bronchospasm |
| Gastrointestinal Disorders | Uncommon | Diarrhoea, nausea, vomiting. |
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicine is important. It allows continued monitoring of the benefit/risk balance of the medicine. Kindly report any suspected adverse reactions to pharmavigil@jbpharma. com
Overdose
Symptoms:
Symptoms are likely to be minor; some abdominal discomfort may be experienced.
Management:
In the event of overdose symptomatic treatment should be given
PHARMACOLOGICAL PROPERTIES
Mechanism of Action
The mode of action of proposed FDC is local and does not depend on absorption into the systemic circulation.
Pharmacodynamic properties
Pharmacotherapeutic classification: A02BX 13. Other drugs for peptic ulcer and gastro-oesophageal reflux disease.
On ingestion the tablet reacts with gastric acid to rapidly form a raft of alginic acid gel having near-neutral pH which floats on the stomach contents effectively impeding gastro-oesophageal reflux for up to 4 hours, and protecting the oesophagus from acid, pepsin and bile. In severe cases the raft itself may be refluxed into the oesophagus in preference to the stomach contents and exert a demulcent effect. In addition in vitro evidence has shown that the raft has a secondary action and is able to entrap bile and pepsin within its structure, further protecting the oesophagus from these gastric components.
Pharmacokinetic properties
The mode of action of Sodium alginate & Potassium bicarbonate tablets is physical and does not depend on absorption into the systemic circulation.
NON CLINICAL PROPERTIES
Animal toxicology or Pharmacology
No preclinical findings relevant to the prescriber have been reported
DESCRIPTION
This product (chewable tablet) contains sodium alginate & bicarbonate and it is Indicated for the treatment of heartburn and indigestion.
Sodium Alginate:
Chemical Name: sodium 3,4,5,6-tetrahydroxyoxane-2-carboxylate Molecular
Formula: (C6H7NaO6)n
Structural Formula:
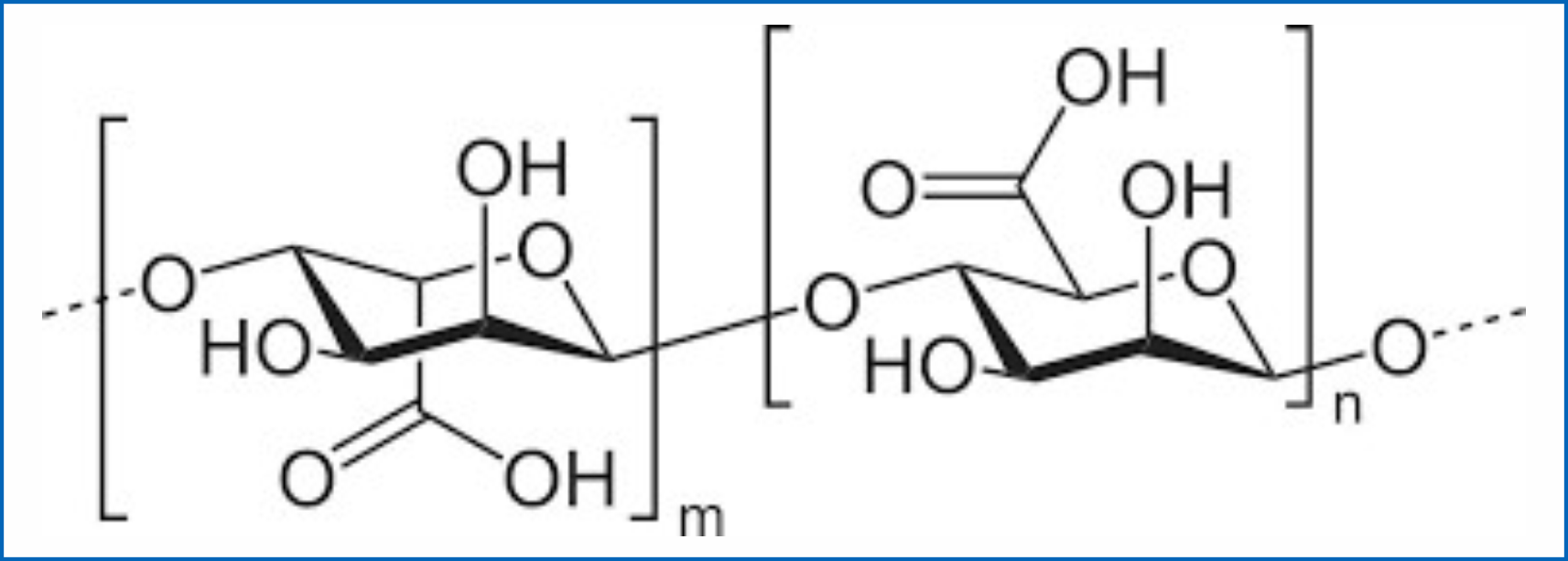
Potassium bicarbonate:
Chemical Name: Potassium hydrogencarbonate
Molecular Formula: KHCO3
Molecular weight: 100.115 g/mol
Structural Formula:
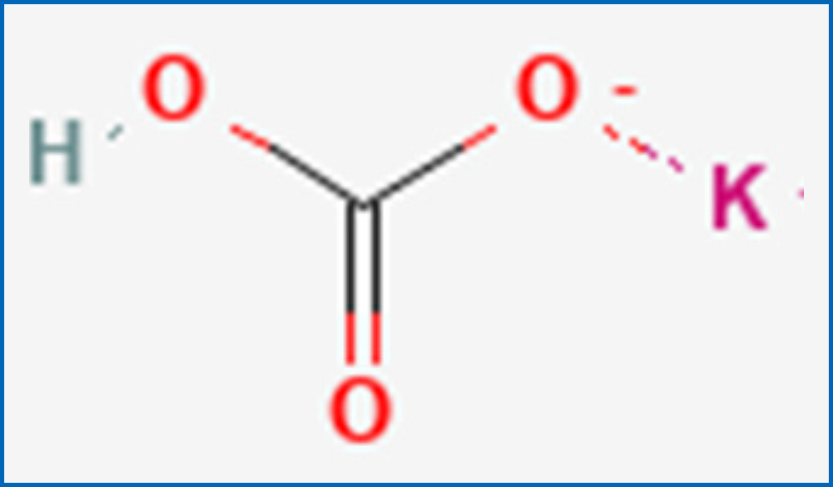
PHARMACEUTICAL PARTICULARS
Incompatibility
Shelf Life
Refer carton
Packaging Information
Blister of 10 tablets
Storage instructions
Store protected from moisture, at a temperature below 30oC.
Keep out of reach of children
PATIENT COUNSELLING INFORMATION
Read all of this leaflet carefully before you start taking this medicine because it contains important information for you.
Keep this leaflet. You may need to read it again.
If you have any further questions, ask your doctor, pharmacist or nurse.
This medicine has been prescribed for you only. Do not pass it on to others. It may harm them, even if their signs of illness are the same as yours.
If you get any side effects, talk to your doctor, pharmacist or nurse. This includes any possible side effects not listed in this leaflet.
Manufactured in India by:
Akums Drugs & Pharmaceuticals Ltd
At: Plot No. 26A, 27-30, Sector-8A, I.I.E., SIDCUL, Ranipur, Haridwar-249 403, Uttarakhand

Note: This prescribing information is applicable for India Market only.
DATE OF REVISION: May 2025